Cell surface carbohydrates play a major role in determining cell-cell interactions in the body. Tumor cells are commonly characterized by the expression of carbohydrate epitopes not found on normal cells or by the overexpression of specific glycans. Changes in carbohydrate composition have been linked to tumor progression in clinical pathology studies. However, it is still unclear when these changes are clinically relevant, whether glycans are good biomarkers in a clinical setting and if enzymes underlying glycan structures are good therapeutic candidates. In addition, understanding of which enzymes correspond to glycan structures observed in cancer is still evolving. My laboratory utilizes systems-based approaches to address these questions. For example, in collaboration with the laboratory of Dr. Rebecca Fitzegerald, we identified glycan-based markers discriminating Barrett’s esophagus, an often benign condition, from Barrett’s dysplasia, 59% of patients with which go on to esophageal cancer (1, Fig. 1). Our recent work has focused on the role of glycosylation in clinical melanoma metastasis, an ongoing collaboration with Dr. Eva Hernando and the Melanoma Research Program in the Perlmutter Cancer Center at NYU-Lagone Medical Center. Although there has been much investigation of glycosylation in melanoma metastasis in mouse model systems, such as the B16 mouse model, the clinical relevance of this work has never been established. In fact, there is very little data on the glycomics of clinical melanoma samples due to the fact that primary melanomas do not give enough material for the standard glycomic approaches (i.e. mass spectrometry). Our work in this area includes the validation of miR-30a/d targeting of GALNT7 as crucial to the pro-metastasis impact of that miRNA (2) and our recent systems-based analysis of primary vs. metastastic melanoma from human clinical samples which revealed a previously unknown glycan driver of melanoma metastasis (recently published in Cancer Cell). Future work in this area includes further exploration of glycan drivers of metastasis and examining the role of glycans in metastatic site selection in clinical melanoma.
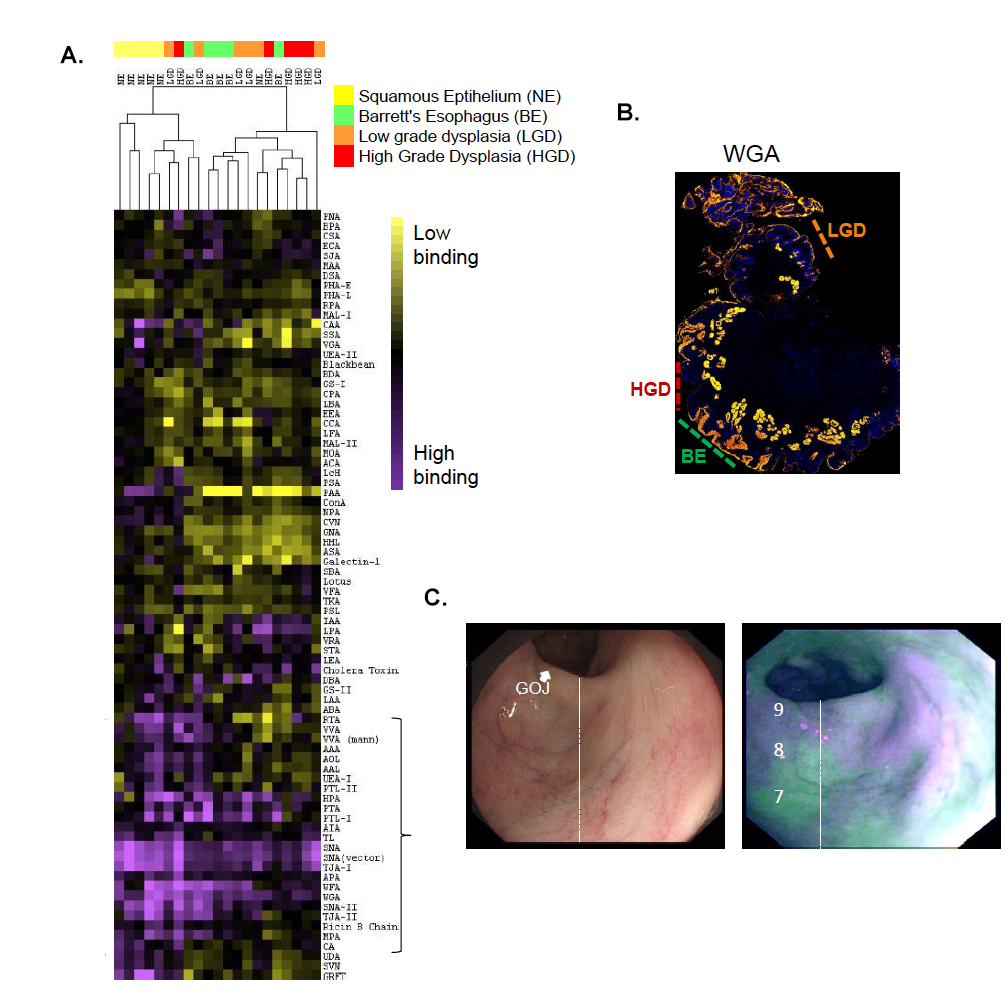
Figure 1. A. Lectin Microarray Analysis of Patient Samples. B. Lectin Histology Confirms WGA Binding Discriminates Barrets Esophagus (BE) from High Grade Dysplasia (HGD). C. WGA Staining of Patient’s Esophagus with Esophageal Cancer shows WGA is a Valid Marker for Endoscopy. Figure adapted from (1).
References
1. Bird-Lieberman, E.L.; Neves, A.A.; Lao-Sirieix, P.; O’Donovan, M.; Novelli, M.; Lovat, L.B.; Eng, W.S.; Mahal, L.K.; Brindle, K.M.; Fitzgerald, R.C. Molecular imaging using fluorescent lectins permits rapid endoscopic identification of dysplasia in Barrett’s esophagus. Nat. Medicine 2012, 18, 315-21.
2. Gaziel-Sovran, A; Segura, M.F.; Di Micco, R; Collins, M.K.; Hanniford, D.; Vega-Saenz de Miera, E.; Rakus, J.F.; Dankert, J.F.; Shang, S.; Kerbel, R.S.; Bhardwaju, N.; Yongzhao, S.; Darvishan, F.; Zavadil, J.; Erlebacher, A.; Mahal, L.K.; Osman, I.; Hernando, E. MiR-30b/30d regulation of GalNAc transferases enhances invasion and immunosuppression during metastasis. Cancer Cell 2011, 20, 104-18.